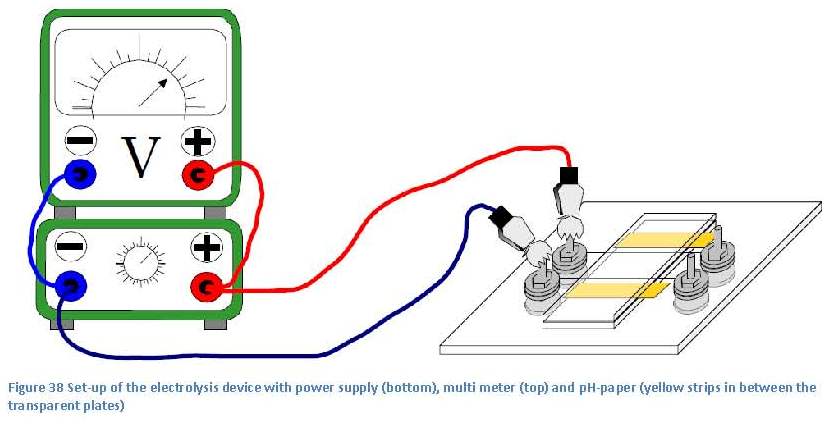
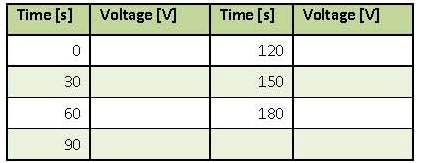
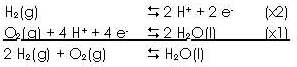Het arrangement 17. Artificial Solar Tree (2011) is gemaakt met Wikiwijs van Kennisnet. Wikiwijs is hét onderwijsplatform waar je leermiddelen zoekt, maakt en deelt.
- Auteurs
- Laatst gewijzigd
- 04-05-2015 09:06:06
- Licentie
-
Dit lesmateriaal is gepubliceerd onder de Creative Commons Naamsvermelding-GelijkDelen 3.0 Nederland licentie. Dit houdt in dat je onder de voorwaarde van naamsvermelding en publicatie onder dezelfde licentie vrij bent om:
- het werk te delen - te kopiëren, te verspreiden en door te geven via elk medium of bestandsformaat
- het werk te bewerken - te remixen, te veranderen en afgeleide werken te maken
- voor alle doeleinden, inclusief commerciële doeleinden.
Meer informatie over de CC Naamsvermelding-GelijkDelen 3.0 Nederland licentie.
Dit materiaal is ontwikkeld door: Redouane Eddeane, Martijn van Gerwen, Joost van Heijst, Ralph Huijgen, Jurgen Jongmans, Jos van Mil & Ruud de Wit in het kader van het vak betadidactiek binnen de lerarenopleiding van de Eindhoven School of Education (TU/e).
De gebruikte afbeeldingen en fragmenten zijn door ons gecontroleerd ten aanzien van hun rechtenvrije beschikbaarheid. Mocht hier onverhoopt toch een probleem blijken, neemt u dan contact op met esoe@tue.nl zodat wij het kunnen herstellen.

Aanvullende informatie over dit lesmateriaal
Van dit lesmateriaal is de volgende aanvullende informatie beschikbaar:
- Toelichting
- This project aims at a transition to a more sustainable way of transportation, by means of a solution that can be implemented in a relatively short time-frame and that is economically feasible.
- Leerniveau
- HAVO 4; VWO 4;
- Leerinhoud en doelen
- Natuur, leven en technologie; NaSk; Scheikunde; Natuurkunde;
- Eindgebruiker
- leerling/student
- Moeilijkheidsgraad
- gemiddeld
- Studiebelasting
- 7 uur 30 minuten
- Trefwoorden
- artificial, beta, duurzaamheid, energie, engels, esoe, natuurkunde, scheikunde, solar, tree, zon

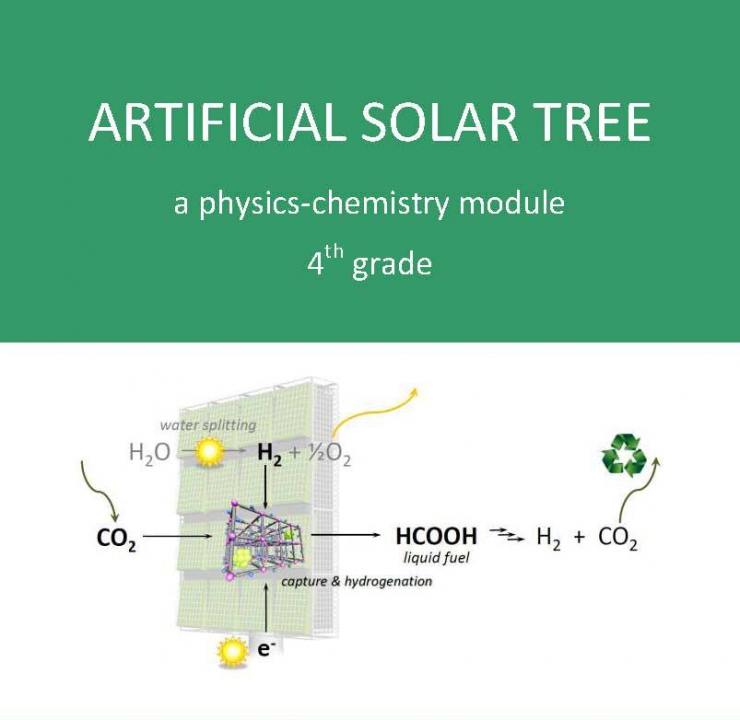
 Global warming
Global warming  facts that Al Gore uses, his movie certainly made the world realize that our planet is in danger.
facts that Al Gore uses, his movie certainly made the world realize that our planet is in danger.
 here every day. So, the blanket around our planet gets thicker and thicker and the Earth gets hotter every day.
here every day. So, the blanket around our planet gets thicker and thicker and the Earth gets hotter every day. and 70 years after that there will not be any coal left either. This, in itself, does not have to be a disaster: since CO2 is emitted when burning these fuels it would be better not to use them at all. However, there has not been found a sustainable way of producing energy that can substitute these fossil fuels. This is exactly the problem that many scientist are working on nowadays.
and 70 years after that there will not be any coal left either. This, in itself, does not have to be a disaster: since CO2 is emitted when burning these fuels it would be better not to use them at all. However, there has not been found a sustainable way of producing energy that can substitute these fossil fuels. This is exactly the problem that many scientist are working on nowadays.


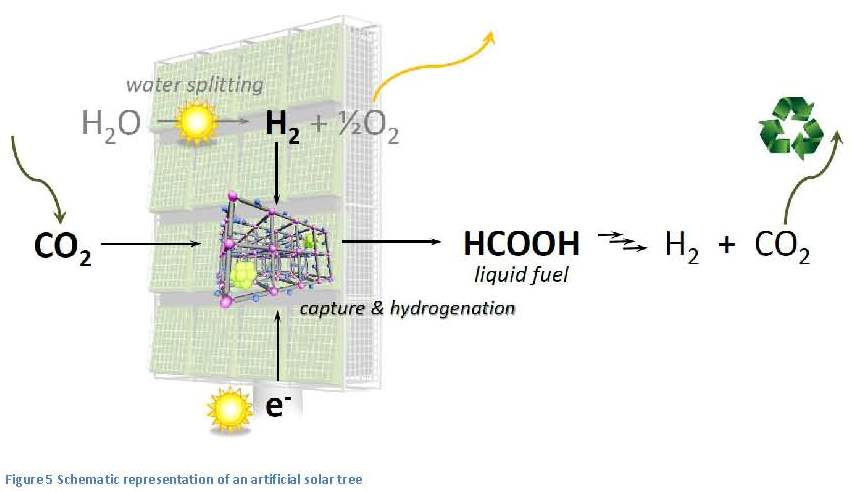
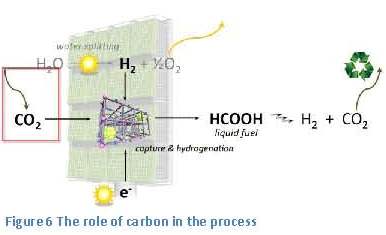
 Introduction
Introduction 
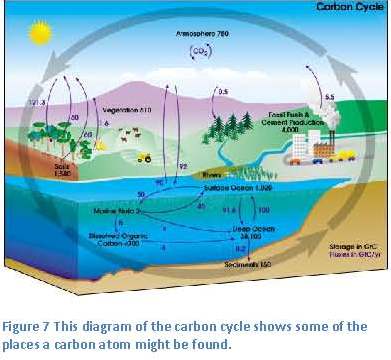
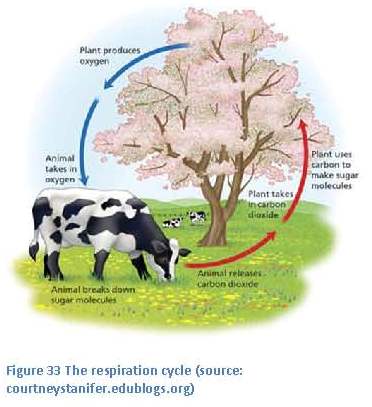
 The amazing transformation that has happened here is changing energy from sunlight into chemical energy that plants and animals can use as food (figure 7). Through the food chain, this carbon moves into all other living things.
The amazing transformation that has happened here is changing energy from sunlight into chemical energy that plants and animals can use as food (figure 7). Through the food chain, this carbon moves into all other living things.
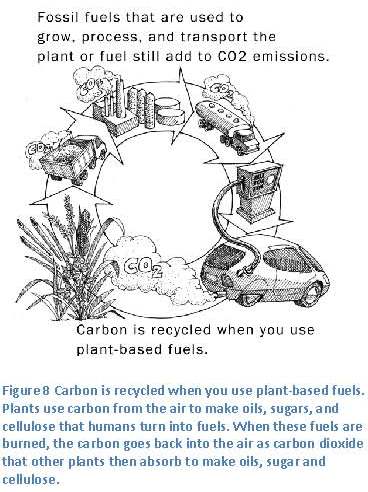




 this assignment, read:
this assignment, read:




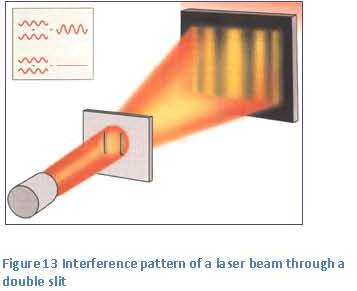
 gravitation we see light as particles and if we want to know the interference pattern we do calculations as if it is a wave.
gravitation we see light as particles and if we want to know the interference pattern we do calculations as if it is a wave.



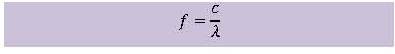
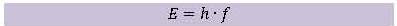

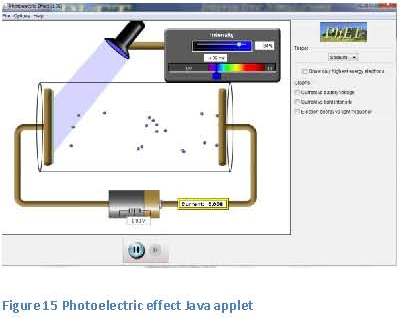
 workings of a photovoltaic cell is given. In order to do that, some new concepts must be introduced. It is not necessary to fully comprehend these concepts or to do complicated calculations. The exercises indicate the level of understanding that is required.
workings of a photovoltaic cell is given. In order to do that, some new concepts must be introduced. It is not necessary to fully comprehend these concepts or to do complicated calculations. The exercises indicate the level of understanding that is required. 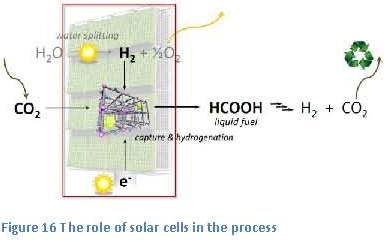









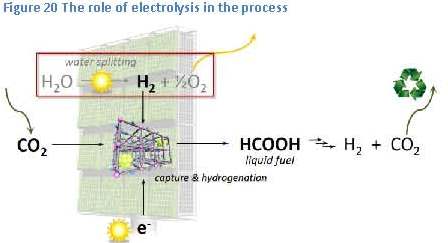
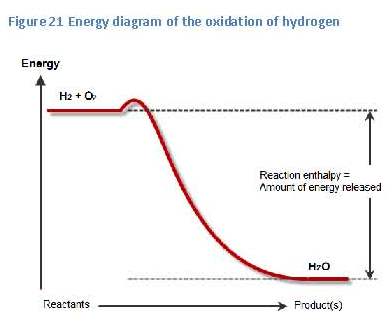









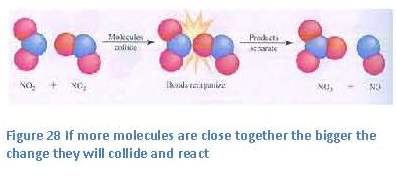



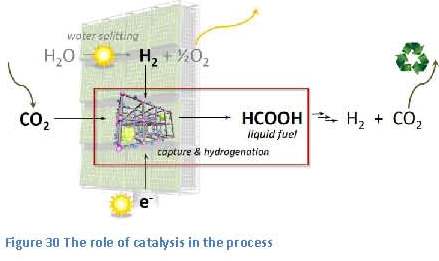 the small bump, or barrier, in the energy-diagram, just to the right of the reactants (see figure 21). The height of this barrier is called “the activation energy”. This activation energy is caused by the higher energy content of the components, or intermediate products, in this so-called transition state of the reaction. Within this transition state the bonds within the reactants have partially been broken and new bonds have partially been formed.
the small bump, or barrier, in the energy-diagram, just to the right of the reactants (see figure 21). The height of this barrier is called “the activation energy”. This activation energy is caused by the higher energy content of the components, or intermediate products, in this so-called transition state of the reaction. Within this transition state the bonds within the reactants have partially been broken and new bonds have partially been formed.





 energy. This is the last part of the overall process, as shown in figure 35. In general two methods are available for this conversion process. These are the internal combustion engine and the fuel cell.
energy. This is the last part of the overall process, as shown in figure 35. In general two methods are available for this conversion process. These are the internal combustion engine and the fuel cell.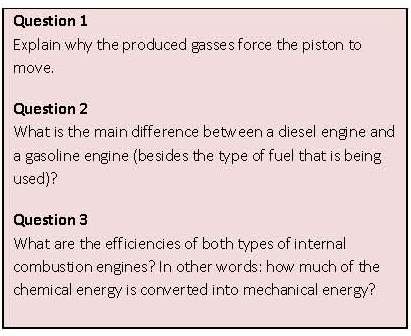
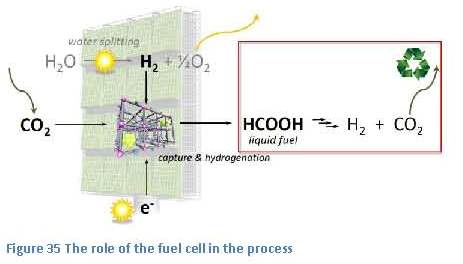
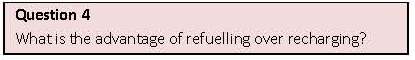
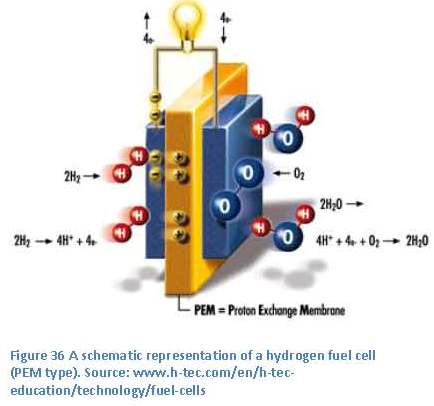 place in this fuel cell are:
place in this fuel cell are: